The Journey of Atomic Discovery: Protons, Electrons, and Neutrons
Written on
Chapter 1: Understanding Atomic Structure
Have you ever wondered how scientists figured out the number of electrons and nucleons in chemical elements, given that no one has ever seen an atom? When we examine the periodic table, we can easily identify the electron count in helium or oxygen, recognize the nucleon number for various elements, and calculate the protons and neutrons in an atom using straightforward math. Yet, these fundamental units of matter—the atoms—remain unseen. They are influential and omnipresent, but their direct observation eludes us. So, how can we ascertain their structure and whether there might be undiscovered components?

To comprehend this enigma, we must journey back to ancient civilizations. In those early days, practitioners of chemistry relied more on ratios and proportions than on precise measurements. Even in Ancient Greece, experiments revealed that the volumes and masses involved in chemical reactions often adhered to whole-number multiples, suggesting a hidden order within the microscopic realm.

As time progressed, scientists began measuring the charge of electrons, yielding values like 4.8, 6.4, 8.0, and 1.6. These values revealed themselves to be multiples of a fundamental constant, specifically 1.6*10^-19. The pivotal realization was that atoms possess mass, with the hydrogen atom recognized as the lightest and subsequently adopted as the standard unit of atomic mass.

Further investigations confirmed that each atom contains an equal number of protons and electrons, with the mass of a hydrogen atom being 1,836 times that of an electron. This led to the understanding that electrons contribute a minimal fraction of the atom's total mass, prompting the evolution of various atomic models over time. Eventually, scientists recognized a mismatch between the atomic number on the periodic table and the mass of atoms, now referred to as the nucleon number, which typically averaged around twice what was expected. This discrepancy gave rise to the hypothesis of a neutral particle, similar in mass to a proton, which was later named the neutron.
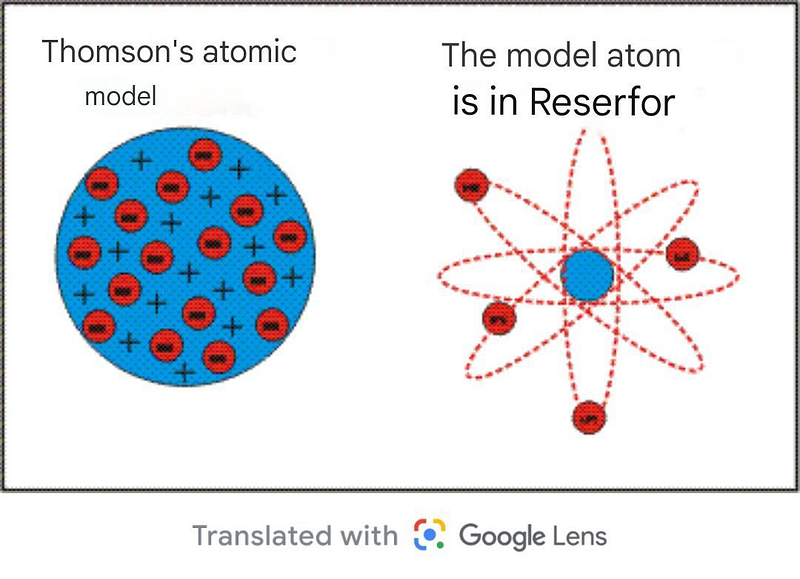
In 1932, James Chadwick confirmed the existence of the neutron, the third critical component of the atom, aligning closely with the previously predicted mass of protons. The count of neutrons in atomic nuclei generally matched the number of protons or differed by just one, confirming earlier theories. Since atoms contain an equal number of protons and electrons, and both, alongside neutrons, account for nearly the entire mass of the atom, we can deduce the number of neutrons by subtracting the proton count from the mass number.
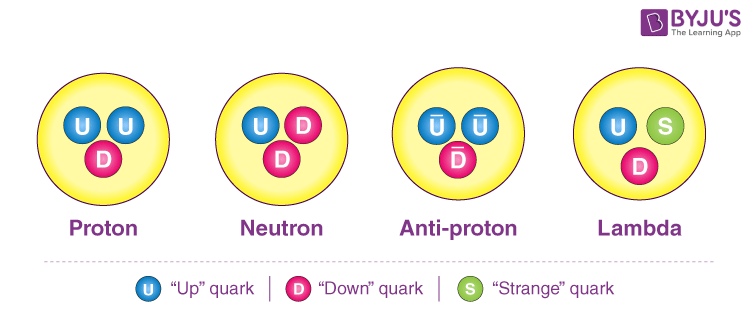
While electrons, protons, and neutrons are often visualized as indivisible spheres, their actual structure is far more intricate. This fascinating topic could be explored in a future article if there is interest!
Please applaud if you'd like to see more articles about space in your feed! Don't forget to subscribe to our channel and submit your questions, which I will address in upcoming articles.
Chapter 2: The Basics of Atomic Composition
This video titled "Atomic Structure: Protons, Electrons & Neutrons" delves into the foundational elements of atomic theory, explaining the roles of protons, electrons, and neutrons in the structure of matter.
The video "Chemistry Science: Protons, Electrons & Neutrons Discovery" outlines the historical journey that led to the discovery of these essential atomic components and their significance in the realm of chemistry.