# Unraveling the Science Behind Pop Rocks: A Fizzy Delight
Written on
Chapter 1: The Allure of Pop Rocks
What creates the delightful fizz in Pop Rocks? Microscopic images reveal the secrets behind this iconic candy!
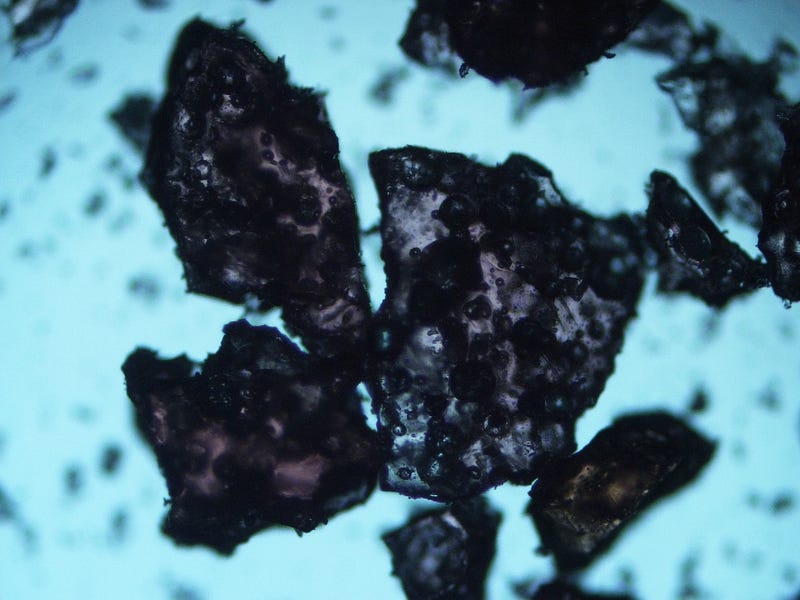
Figure 1. Pop Rocks candy magnified, showcasing various fragment sizes. The larger pieces are prominently displayed.
Pop Rocks were undoubtedly the most entertaining candy during my childhood. Sharing them with friends turned into a light-hearted contest to see who could fit the most in their mouths for the ultimate fizzing experience. The sensation of those tiny explosions on my tongue was simply hilarious as a child. Now, as an adult and food scientist, my interest lies in understanding the intriguing process behind the creation of this unique candy and what generates its popping effect.
The invention of Pop Rocks is credited to William Mitchell, a research chemist at General Foods during the late 1950s (which later became part of Kraft-Heinz in the 1990s). Like many great discoveries, Pop Rocks were born from an accident. Mitchell was experimenting with creating an instant soda tablet when he inadvertently developed a hard candy that fizzed and popped in the mouth.
The creation of Pop Rocks begins with a blend of sucrose, lactose, and corn syrup. A small amount of water is introduced to dissolve the crystalline sugars, transforming the mixture into syrup. This syrup is then heated to temperatures between 290-310°F to evaporate most of the water. For context, when making jelly or marshmallow candy, the maximum cooking temperature is typically around 230-235°F. However, with Pop Rocks, a moisture content of only 2% is desired, necessitating prolonged heating at high temperatures.
After sufficient water has been evaporated, the hot syrup is transferred to a pressure chamber where carbon dioxide gas is injected. Achieving the right viscosity of the syrup is crucial for incorporating small CO2 bubbles. If the syrup cools too much, it becomes too viscous for effective bubbling; if it's too fluid, the gas forms large bubbles instead of tiny ones.
To maintain the proper viscosity, the pressure chamber is kept at approximately 240°F. The syrup is agitated while under pressurized CO2 gas to create gas bubbles within the mixture. The CO2 is maintained at around 600 psi—about 20 times the pressure in a mountain bike tire and 100 times that of a standard beer can.
Once under pressure, the syrup is moved to a second container, where it cools rapidly, permanently trapping the high-pressure CO2 bubbles within its structure. This swift cooling is essential, as it forces the candy into a glassy state rather than allowing it to crystallize.
Following solidification, the second vessel undergoes a “shock treatment,” which is essentially a worker using a sledgehammer to shatter the candy glass into tiny pieces. As the pressure is released, the vessel can be opened, allowing the candy to fall out.
The key to the iconic pop, hiss, and fizz of this candy lies in the entrapment of those pressurized CO2 bubbles within the sugar glass. Under a microscope, these tiny bubbles can be observed, particularly in the transparent areas of the candy. Circles have been added to the micrograph below to highlight the visible CO2 bubbles.
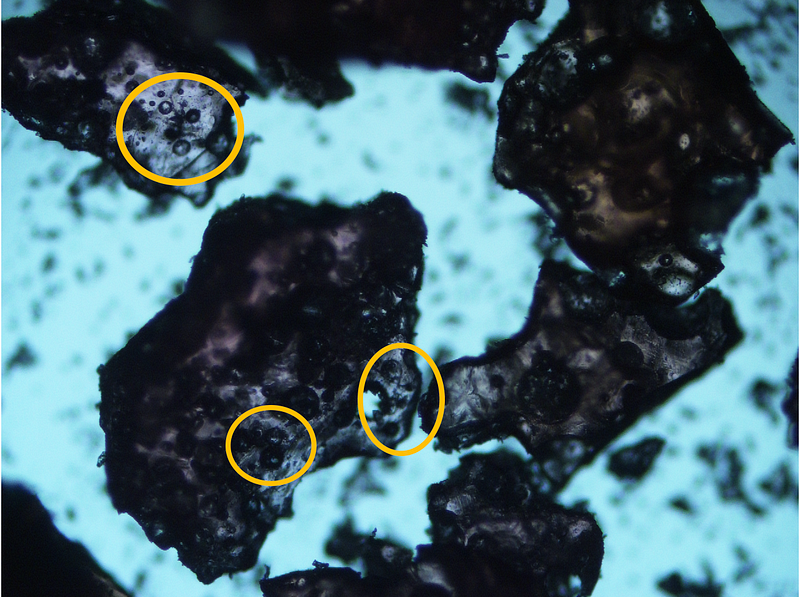
Figure 2. Magnified view showing small carbon dioxide bubbles within the sugar glass, with yellow circles to aid identification.
Indeed, some candies and foods are classified as glasses, which means their molecules are randomly arranged, often due to rapid cooling that doesn't allow for an ordered structure. This results in a product that behaves like a solid for a period of time.
If you're struggling to visualize what an edible glass is, consider the stunts you see in movies where actors crash through windows. Those are not real glass windows; instead, they use sugar glass made from a corn syrup and sucrose mixture that has been set into a glassy state. Since no coloring agents are used, the sugar glass remains clear and shatters like real glass—though it seems a waste of candy, it does prevent injuries.
In contrast to glassy candies, those that cool more slowly tend to become crystalline. Crystalline candies possess an organized, lattice structure, allowing the molecules to align themselves favorably as the crystal grows. Examples of crystalline candies include rock candy, tablets, and powdered candies, which tend to be more stable compared to glasses that absorb moisture and dissolve easily.
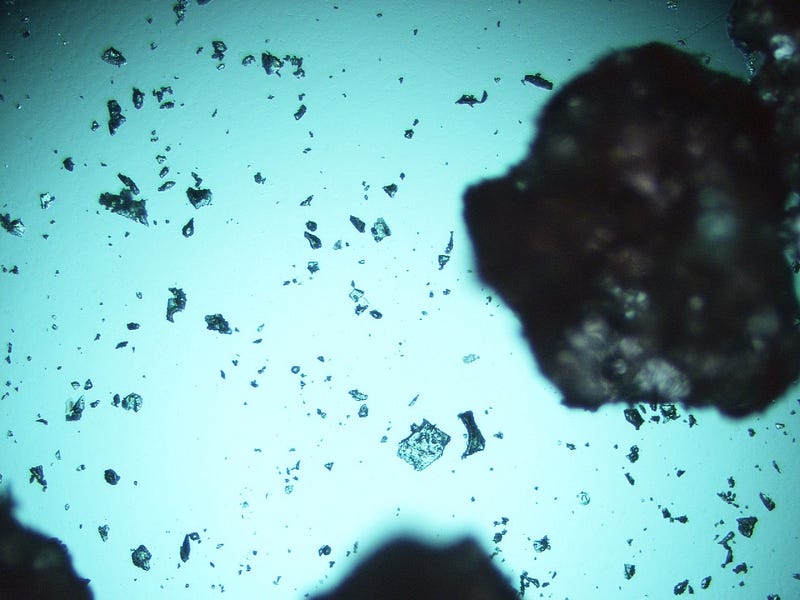
Figure 3. The “shock treatment” results in irregular particle sizes, producing fragments of various dimensions.
As a distinct and whimsical treat, Pop Rocks have faced skepticism and concern from the public. There were rumors that consuming Pop Rocks alongside soda could lead to stomach explosions. Those familiar with the show MythBusters will recall their first episode debunking this myth using a pig stomach. Nevertheless, parental concern grew so significant that Mitchell was sent on a tour by his food company to alleviate public anxiety. The FDA even established a hotline for worried parents regarding the safety of Pop Rocks.
Though always a favorite among children, Pop Rocks have recently gained popularity as a trendy ingredient in upscale food and beverages. I've seen them used to rim cocktails and margaritas, as well as incorporated into meat and foie gras dishes. Chocolate bars featuring Pop Rocks create a fascinating sensory experience.
Even if Pop Rocks aren't your favorite, you can't deny their uniqueness. How many treats sound like a mini fireworks display in your mouth? The sounds are so pronounced that friends and family can hear the popping when you open your mouth wide. The resurgence of Pop Rocks in restaurant cuisine evokes a sense of nostalgia for many adults, myself included.